Metastatic
which means the cancer has spread to other parts of the body
Not actual patient.
Melanoma is considered an aggressive type of skin cancer. It develops when healthy cells called melanocytes start to grow out of control. Melanocytes are the cells that give your skin its pigment, or color.
There are characteristics that may be specific to someone’s diagnosis. Melanoma can be:
which means the cancer has spread to other parts of the body
which means the cancer cannot be removed by surgery
A few factors can determine the stage of melanoma, including the tumor size, if it’s ulcerated, and where it has spread.
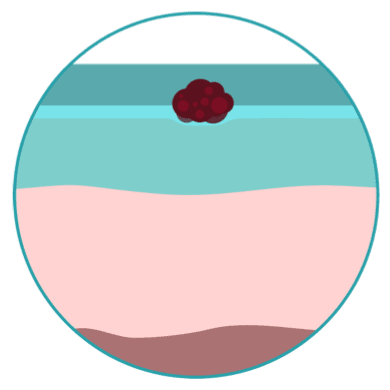
STAGE
0
This is the earliest stage. Cancer cells are only found in the outer, or first, layer of skin called the epidermis.

STAGE
1
The melanoma cells are in the first layer of skin, as well as the second layer of skin called the dermis. The tumor thickness, or how deeply it has penetrated the skin, is up to 2mm thick. The tumor may or may not have ulcerated.

STAGE
2
The cancer cells are in the first and second layers of skin. The following factors are used to determine if the cancer is Stage 2: The thickness of the tumor and if it has ulcerated.

STAGE
3
The melanoma cells have spread locally to nearby lymph nodes and/or to very small areas of nearby skin. The following factors are used to determine if the cancer is Stage 3: The tumor thickness, the number of lymph nodes the cancer cells have spread to, and if the tumor has ulcerated.
STAGE
4
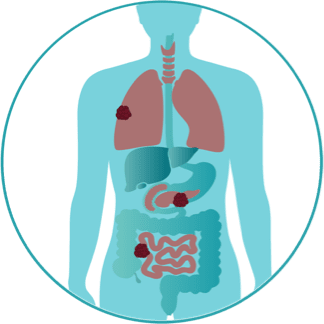
The melanoma has spread to other parts of the body. The most common sites are distant skin and lymph nodes, then lungs, liver, brain, bone, and/or intestines. This is when the cancer is metastatic.
If your melanoma is metastatic, that means the cancer has spread to other parts of the body. There are certain characteristics that may be specific to someone’s diagnosis. One of these specific characteristics can be identified by something called a “biomarker.”

Found by testing tissue from the tumor or a sample of blood.
Used to help doctors know if the cancer has an abnormal gene that can cause cancer to grow and spread faster.
Knowing if your cancer has abnormal genes from biomarker testing can help your doctor recommend treatment options.
Talk to your doctor about any test results that could help you better understand your diagnosis.
One common biomarker is called “BRAF.” Of all people with metastatic melanoma, about half tested BRAF positive (BRAF+). This means the cancer has a certain type of abnormal BRAF (V600E or V600K) gene. Your doctor may recommend a treatment combination based on your test results and diagnosis.
Not actual patients.
No matter where you are in your treatment journey, there are organizations that you can go to for support. The organizations below may help you feel a sense of community with people in a similar situation and may also provide you with useful information.
AIM at Melanoma
Melanoma Research Alliance
Melanoma Research Foundation
Pfizer Inc. does not control or endorse third-party organizations. The content provided by Pfizer Inc. or these organizations is meant for informational purposes only. It is not meant to replace your doctor’s medical advice.
BRAFTOVI + MEKTOVI | See how BRAFTOVI (encorafenib) + MEKTOVI (binimetinib) works. | How it workS |
IMPORTANT SAFETY INFORMATION AND USE
FIND OUT MORE
COLLAPSE
BRAFTOVI (encorafenib) and MEKTOVI (binimetinib) are prescription medicines used together to treat adults with a type of skin cancer called melanoma:
BRAFTOVI should not be used to treat people with wild-type BRAF melanoma. Your healthcare provider will perform a test to make sure that BRAFTOVI + MEKTOVI is right for you.
It is not known if BRAFTOVI or MEKTOVI is safe and effective in children.
BRAFTOVI (encorafenib) and MEKTOVI (binimetinib) may cause serious side effects, including:
Check your skin and tell your healthcare provider right away about any skin changes, including a:
Your healthcare provider should check your skin before treatment, every 2 months during treatment, and for up to 6 months after you stop treatment to look for any new skin cancers.
Your healthcare provider should also check for cancers that may not occur on the skin. Tell your healthcare provider about any new symptoms that develop during treatment.
Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with BRAFTOVI and MEKTOVI if you have certain side effects.
Tell your healthcare team if you are pregnant or plan to become pregnant. BRAFTOVI and MEKTOVI can harm your unborn baby.
Talk to your healthcare team if you are breastfeeding or plan to breastfeed. It is not known if either treatment passes into your breast milk. Do not breastfeed during treatment with BRAFTOVI and MEKTOVI and for:
BRAFTOVI may cause fertility problems in males. This may affect your ability to father a child. Talk to your healthcare provider if this is a concern for you.
The most common side effects of BRAFTOVI when taken with MEKTOVI include: fatigue, nausea, diarrhea, vomiting, stomach-area (abdominal) pain, and pain or swelling of your joints (arthralgia).
Before taking BRAFTOVI + MEKTOVI, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. BRAFTOVI and certain other medicines can affect each other, causing side effects or affecting how BRAFTOVI or other medicines work. You should also avoid grapefruit products during treatment with BRAFTOVI.
These are not all of the possible side effects of BRAFTOVI and MEKTOVI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch. You may also report side effects to Pfizer Inc. at 1-800-438-1985.
BRAFTOVI (encorafenib) and MEKTOVI (binimetinib) are prescription medicines used together to treat adults with a type of skin cancer called melanoma:
BRAFTOVI should not be used to treat people with wild-type BRAF melanoma. Your healthcare provider will perform a test to make sure that BRAFTOVI + MEKTOVI is right for you.
It is not known if BRAFTOVI or MEKTOVI is safe and effective in children.
Please see both BRAFTOVI full Prescribing Information, including Medication Guide, and MEKTOVI full Prescribing Information, including Medication Guide, for additional information.
BRAFTOVI (encorafenib) is a prescription medicine used in combination with medicines called cetuximab and mFOLFOX6 (fluorouracil, leucovorin, and oxaliplatin) to treat people with cancer of the colon or rectum (colorectal cancer):
BRAFTOVI in combination with cetuximab and mFOLFOX6 was approved based on response rate and how long patients’ responses lasted. There is ongoing evaluation of clinical benefit of BRAFTOVI in combination with cetuximab and mFOLFOX6.
BRAFTOVI is a prescription medicine used in combination with a medicine called cetuximab to treat adults with cancer of the colon or rectum (colorectal cancer) after past treatment:
BRAFTOVI should not be used to treat people with wild-type BRAF colorectal cancer. Your healthcare provider will perform a test to make sure that BRAFTOVI is right for you.
It is not known if BRAFTOVI is safe and effective in children.
BRAFTOVI (encorafenib) is a prescription medicine used in combination with medicines called cetuximab and mFOLFOX6 (fluorouracil, leucovorin, and oxaliplatin) to treat people with cancer of the colon or rectum (colorectal cancer):
BRAFTOVI in combination with cetuximab and mFOLFOX6 was approved based on response rate and how long patients’ responses lasted. There is ongoing evaluation of clinical benefit of BRAFTOVI in combination with cetuximab and mFOLFOX6.
BRAFTOVI is a prescription medicine used in combination with a medicine called cetuximab to treat adults with cancer of the colon or rectum (colorectal cancer) after past treatment:
BRAFTOVI should not be used to treat people with wild-type BRAF colorectal cancer. Your healthcare provider will perform a test to make sure that BRAFTOVI is right for you.
It is not known if BRAFTOVI is safe and effective in children.
BRAFTOVI is a prescription medicine used:
BRAFTOVI in combination with cetuximab and mFOLFOX6 was approved based on response rate and how long patients’ responses lasted. There is ongoing evaluation of clinical benefit of BRAFTOVI in combination with cetuximab and mFOLFOX6.
BRAFTOVI should not be used to treat people with wild-type BRAF melanoma, wild-type BRAF colorectal cancer, or wild-type BRAF NSCLC. Your healthcare provider will perform a test to make sure that BRAFTOVI or BRAFTOVI with MEKTOVI is right for you.
Your healthcare provider will perform a test to make sure that BRAFTOVI or BRAFTOVI with MEKTOVI is right for you.
It is not known if BRAFTOVI or MEKTOVI is safe and effective in children.
BRAFTOVI (encorafenib) is a prescription medicine used:
BRAFTOVI in combination with cetuximab and mFOLFOX6 was approved based on response rate and how long patients’ responses lasted. There is ongoing evaluation of clinical benefit of BRAFTOVI in combination with cetuximab and mFOLFOX6.
BRAFTOVI should not be used to treat people with wild-type BRAF melanoma, wild-type BRAF colorectal cancer, or wild-type BRAF NSCLC. Your healthcare provider will perform a test to make sure that BRAFTOVI or BRAFTOVI with MEKTOVI is right for you.
Your healthcare provider will perform a test to make sure that BRAFTOVI or BRAFTOVI with MEKTOVI is right for you.
It is not known if BRAFTOVI or MEKTOVI is safe and effective in children.
BRAFTOVI (encorafenib) is a prescription medicine used:
BRAFTOVI and MEKTOVI are prescription medicines used together to treat adults with a type of skin cancer called melanoma:
BRAFTOVI should not be used to treat people with wild-type BRAF melanoma. Your healthcare provider will perform a test to make sure that BRAFTOVI + MEKTOVI is right for you.
It is not known if BRAFTOVI or MEKTOVI is safe and effective in children.
BRAFTOVI (encorafenib) and MEKTOVI (binimetinib) are prescription medicines used together to treat adults with a type of skin cancer called melanoma:
BRAFTOVI should not be used to treat people with wild-type BRAF melanoma. Your healthcare provider will perform a test to make sure that BRAFTOVI + MEKTOVI is right for you.
It is not known if BRAFTOVI or MEKTOVI is safe and effective in children.
BRAFTOVI and MEKTOVI are prescription medicines used together to treat adults with a type of lung cancer called non-small cell lung cancer (NSCLC):
BRAFTOVI (encorafenib) is a prescription medicine used:
BRAFTOVI should not be used to treat people with wild-type BRAF NSCLC. Your healthcare provider will perform a test to make sure that BRAFTOVI + MEKTOVI is right for you.
It is not known if BRAFTOVI or MEKTOVI is safe and effective in children.
BRAFTOVI (encorafenib) and MEKTOVI (binimetinib) are prescription medicines used together to treat adults with a type of lung cancer called non-small cell lung cancer (NSCLC):
BRAFTOVI should not be used to treat people wild-type BRAF NSCLC. Your healthcare provider will perform a test to make sure that BRAFTOVI with MEKTOVI is right for you.
It is not known if BRAFTOVI or MEKTOVI is safe and effective in children.